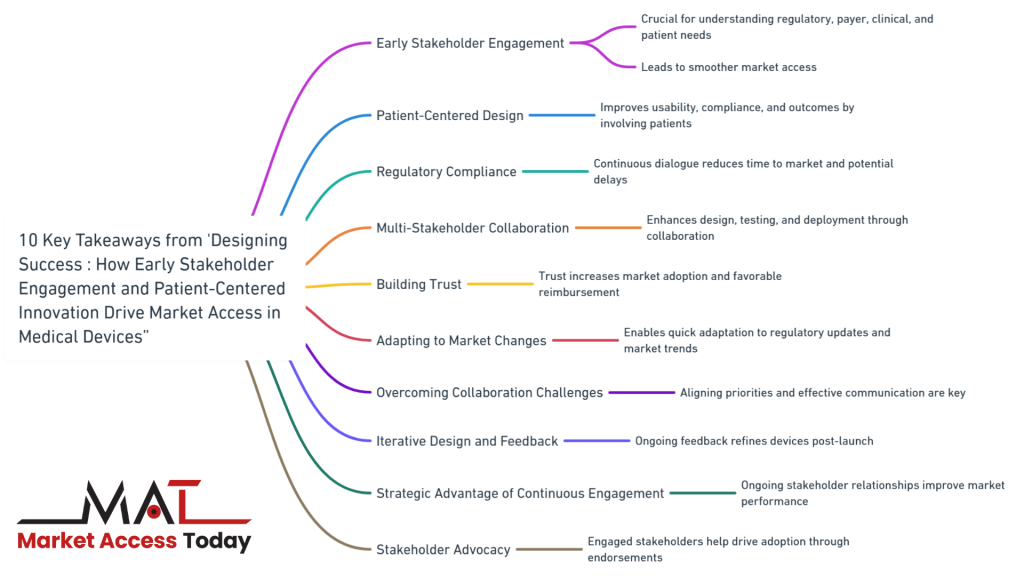
10 Key Takeaways from “Designing Success: How Early Stakeholder Engagement and Patient-Centered Innovation Drive Market Access in Medical Devices”
- Early Stakeholder Engagement: Engaging with stakeholders early in the medical device development process is crucial for understanding regulatory requirements, payer expectations, clinical needs, and patient preferences, leading to smoother market access.
- Patient-Centered Design: Involving patients in the design and evaluation phases ensures that medical devices meet their needs, improving usability, compliance, and overall patient outcomes.
- Regulatory Compliance: Continuous dialogue with regulatory bodies helps manufacturers stay informed about evolving requirements, reducing time to market and avoiding potential delays.
- Multi-Stakeholder Collaboration: Collaborating with regulators, payers, clinicians, and patients from the outset enhances the design, testing, and deployment of medical devices, ensuring they meet the needs of all parties involved.
- Building Trust: Establishing trust with payers and clinicians through early and continuous engagement increases the likelihood of market adoption and favorable reimbursement decisions.
- Adapting to Market Changes: Continuous engagement with stakeholders enables manufacturers to quickly adapt to regulatory updates and shifting market trends, maintaining competitiveness and compliance.
- Overcoming Collaboration Challenges: Effective communication, aligning stakeholder priorities, and managing complexity are essential for successful multi-stakeholder collaboration.
- Iterative Design and Feedback: An iterative design process, informed by ongoing patient and clinician feedback, helps manufacturers refine devices post-launch, ensuring they remain relevant and effective.
- Strategic Advantage of Continuous Engagement: Maintaining ongoing relationships with stakeholders throughout the device lifecycle provides strategic benefits, including improved market performance and long-term success.
- Stakeholder Advocacy: Engaged stakeholders are more likely to become advocates for a device, helping to drive broader market adoption through positive endorsements and testimonials.
Please look at the Market Access & HEOR Resource category for more articles.
The Evolving Landscape of Medical Devices
The medical devices industry is undergoing a profound transformation, driven by rapid advancements in technology, changing regulatory landscapes, and increasing demand for innovative solutions that improve patient outcomes.
In this competitive and highly regulated environment, successfully bringing a new medical device to market requires more than just cutting-edge technology—it demands a deep understanding of the needs and expectations of all stakeholders involved. From regulators and payers to clinicians and, most importantly, patients, the pathway to market access is now paved with collaboration and continuous engagement.
Why Stakeholder Engagement Matters
In the past, the development of medical devices often followed a linear path: design, testing, approval, and then market launch. However, this traditional approach is no longer sufficient in today’s dynamic healthcare ecosystem. The complexity of modern medical devices, coupled with the diverse requirements of different stakeholders, has led to a shift towards more integrated and iterative development processes. Engaging stakeholders early and maintaining that engagement throughout the product lifecycle has become crucial for navigating regulatory hurdles, securing reimbursement, and ensuring clinical adoption.
Early and continuous engagement with stakeholders allows manufacturers to gather valuable insights that can shape the development of a device, from design and functionality to pricing and market positioning. By understanding the specific needs of regulators, payers, clinicians, and patients, manufacturers can align their strategies to meet these expectations, ultimately facilitating smoother and faster market access. Moreover, involving patients in the design and evaluation phases ensures that the final product truly addresses their needs and improves their quality of life, leading to higher adoption rates and better overall outcomes.
This article explores the critical importance of early and continuous stakeholder engagement in the development of medical devices. We will delve into the role of multi-stakeholder collaboration, the impact of patient-centered design, and how these strategies are not just beneficial but essential for achieving successful market access. Whether you are a manufacturer looking to bring a new device to market or a healthcare professional interested in the future of medical device innovation, understanding these trends is key to staying ahead in this ever-evolving industry.
The Role of Early and Continuous Stakeholder Engagement
In today’s rapidly evolving medical device industry, the traditional approach to product development—where stakeholder engagement often occurred late in the process—has given way to a more collaborative and integrated model. Engaging with stakeholders early and maintaining that engagement throughout the device’s lifecycle is now recognized as a critical factor in achieving successful market access. This section delves into why this approach is essential and how it benefits the development process.
Understanding Stakeholder Needs
The medical devices landscape is populated by a diverse group of stakeholders, each with their own priorities, expectations, and concerns. These stakeholders include regulators who are focused on safety and efficacy, payers concerned with cost-effectiveness and reimbursement, clinicians interested in clinical utility and ease of use, and patients whose lives will be directly impacted by the device.
Regulators:
Regulators are the gatekeepers of the medical devices market, ensuring that all products meet stringent safety and efficacy standards before they can be used by patients. By engaging with regulators early in the development process, manufacturers can gain critical insights into the regulatory requirements specific to their device. This early dialogue helps manufacturers design their clinical trials and compile data in a way that aligns with regulatory expectations, potentially shortening the time to market and reducing the risk of costly delays.
Payers:
Payers, including insurance companies and government health programs, are increasingly focused on value-based care. They need to be convinced that a new device not only improves patient outcomes but also offers a cost-effective solution compared to existing treatments. Early engagement with payers allows manufacturers to understand the economic models and data that will be required to justify reimbursement. This can lead to the development of a pricing strategy that aligns with payer expectations and increases the likelihood of favorable reimbursement decisions.
Clinicians:
Clinicians are on the front lines of patient care, and their acceptance of a new device is crucial for its success in the market. Engaging with clinicians early enables manufacturers to gather feedback on the device’s design, usability, and clinical applicability. This feedback can be invaluable in refining the product to better meet the needs of healthcare professionals, ensuring that it fits seamlessly into existing workflows and ultimately improving patient care.
Patients:
Patients are increasingly recognized as key stakeholders in the development of medical devices. Their perspectives on usability, comfort, and impact on quality of life are critical in ensuring that the final product meets their needs. Early and continuous engagement with patients through focus groups, surveys, and usability testing helps manufacturers design devices that are not only clinically effective but also user-friendly and aligned with patient preferences.
Facilitating Regulatory Compliance
One of the most significant benefits of early stakeholder engagement is the ability to navigate the complex regulatory environment more effectively. Each regulatory body, whether it’s the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), or other regional regulators, has its own set of requirements and expectations for medical devices. Engaging with these bodies early in the development process allows manufacturers to identify potential regulatory hurdles and address them proactively.
Proactive Dialogue with Regulators:
By establishing a dialogue with regulators early, manufacturers can gain clarity on the specific data and documentation that will be required for approval. This includes understanding the types of clinical trials that will be necessary, the endpoints that need to be measured, and the post-market surveillance requirements that will be imposed. Early engagement also provides an opportunity to discuss innovative technologies or novel device features that may not fit neatly into existing regulatory frameworks, potentially leading to the development of new guidelines or pathways for approval.
Reducing Time to Market:
Engaging with regulators throughout the development process helps to ensure that there are no surprises or significant delays when it comes time to submit the device for approval. Manufacturers can receive early feedback on their clinical trial designs, regulatory submissions, and risk management plans, allowing them to make adjustments before finalizing their applications. This proactive approach can significantly reduce the time to market, providing a competitive advantage in the fast-paced medical devices industry.
Building Trust with Payers and Clinicians
For a medical device to succeed in the market, it must gain the trust and confidence of both payers and clinicians. Early and continuous engagement with these stakeholders is key to achieving this goal.
Demonstrating Value to Payers:
Payers are looking for devices that provide tangible value—whether through improved patient outcomes, reduced healthcare costs, or enhanced efficiency in care delivery. By engaging with payers early, manufacturers can understand the specific criteria that will be used to assess the device’s value. This understanding allows manufacturers to design clinical trials that not only demonstrate safety and efficacy but also provide robust data on the economic impact of the device. In turn, this data can be used to build a compelling case for reimbursement, increasing the likelihood of payer acceptance and broad market access.
Securing Clinical Buy-In:
Clinicians play a critical role in the adoption of new medical devices. If a device is not user-friendly, does not integrate well into existing workflows, or does not clearly demonstrate clinical benefits, it is unlikely to gain traction in the marketplace. Engaging with clinicians early in the development process provides manufacturers with insights into the real-world challenges faced by healthcare providers. By incorporating this feedback into the design and development of the device, manufacturers can create products that not only meet clinical needs but also enhance the overall patient care experience.
Clinical Advisory Boards and Early Trials:
Forming clinical advisory boards or conducting early clinical trials in collaboration with key opinion leaders (KOLs) can be an effective way to build credibility and trust among the broader clinical community. These collaborations allow manufacturers to gather valuable insights, refine their devices based on expert feedback, and create advocates who can champion the device once it enters the market.

Multi-Stakeholder Collaboration: A Key to Market Access
In the modern medical device industry, the concept of collaboration has evolved from a “nice-to-have” to a “must-have” strategy. Multi-stakeholder collaboration, which involves regulators, payers, clinicians, patients, and other key players, is essential for navigating the complex landscape of market access. This section explores how collaboration across these diverse groups not only streamlines the path to market but also ensures that medical devices are more effectively designed, tested, and deployed.
Collaborative Development Process
In the traditional model of medical device development, manufacturers often worked in silos, developing products with minimal input from external stakeholders. However, this approach can lead to delays, regulatory setbacks, and devices that may not fully meet the needs of end users. By contrast, a collaborative development process involves engaging with multiple stakeholders from the earliest stages of product development, ensuring that their insights and expertise inform every step of the process.
Early Input from Regulators:
One of the first steps in a collaborative development process is seeking early input from regulatory bodies. This collaboration helps manufacturers understand the specific requirements and expectations that regulators have for the device in question. By involving regulators early, manufacturers can design their clinical trials and gather the necessary data to meet these requirements, reducing the risk of regulatory delays or rejections.
Involvement of Clinicians and Healthcare Providers:
Clinicians and healthcare providers bring invaluable insights into how a device will be used in real-world settings. By collaborating with these stakeholders during the design phase, manufacturers can identify potential challenges related to usability, workflow integration, and patient safety. This input allows manufacturers to refine the device, making it more intuitive and effective for clinical use. Moreover, involving clinicians in the development process can lead to the creation of clinical advisory boards, where experts can provide ongoing feedback and support the device’s introduction to the market.
Payers’ Perspective on Value and Reimbursement:
Engaging with payers early in the development process is crucial for understanding the economic considerations that will influence the device’s market access. Payers are focused on value-based care, meaning they want to see evidence that a new device improves patient outcomes and reduces overall healthcare costs. Collaborative discussions with payers can help manufacturers design studies that not only demonstrate clinical efficacy but also highlight cost-effectiveness and other factors that will support favorable reimbursement decisions.
Patient Involvement in Design and Evaluation:
Patients are the ultimate end-users of medical devices, and their feedback is critical to the device’s success. By involving patients in the design and evaluation phases, manufacturers can ensure that the device meets their needs, is user-friendly, and improves their quality of life. Patient input can lead to innovations that may not have been considered otherwise, such as adjustments for comfort, ease of use, and accessibility. This collaboration also fosters patient trust and acceptance, which are key to successful market adoption.
Overcoming Challenges in Collaboration
While multi-stakeholder collaboration offers significant benefits, it is not without its challenges. Differences in priorities, communication barriers, and the complexity of coordinating multiple groups can pose obstacles. However, these challenges can be overcome with careful planning, clear communication, and a commitment to collaboration.
Aligning Stakeholder Priorities:
One of the primary challenges in multi-stakeholder collaboration is aligning the priorities of different groups. For example, while clinicians may focus on clinical efficacy and ease of use, payers are more concerned with cost-effectiveness and long-term value. To address this, manufacturers should facilitate open discussions where stakeholders can voice their concerns and priorities. By understanding each group’s perspective, manufacturers can find common ground and develop a product that meets the needs of all parties involved.
Effective Communication Strategies:
Communication barriers can hinder collaboration, particularly when stakeholders come from diverse backgrounds with different areas of expertise. To overcome this, manufacturers should establish clear communication channels and use language that is accessible to all stakeholders. Regular updates, transparent decision-making processes, and opportunities for feedback are essential components of effective communication. Additionally, appointing a project manager or liaison to coordinate between stakeholders can help ensure that everyone stays informed and engaged.
Managing Complexity:
Coordinating a multi-stakeholder collaboration can be complex, especially as the number of stakeholders increases. Manufacturers should develop a structured plan that outlines the roles and responsibilities of each stakeholder, along with timelines and milestones for the project. Utilizing project management tools and technologies can help streamline the process, making it easier to track progress and manage the various moving parts.

Patient-Centered Design and Feedback for Market Access
In the development of medical devices, one of the most significant shifts in recent years has been the move towards patient-centered design. This approach places patients at the heart of the design and development process, ensuring that their needs, preferences, and experiences directly influence the final product. Involving patients early and consistently throughout the development cycle not only enhances the usability and effectiveness of medical devices but also plays a critical role in their successful market access and adoption.
The Shift Towards Patient-Centered Innovation
Traditionally, medical devices were often developed with a primary focus on clinical efficacy and the needs of healthcare providers, with patients considered more as end-users than active participants in the design process. However, the landscape has changed dramatically as the importance of patient experiences and outcomes has become more apparent.
Understanding Patient Needs:
Patient-centered design begins with a deep understanding of the needs, challenges, and daily experiences of patients who will ultimately use the device. This understanding goes beyond clinical data to encompass the physical, emotional, and psychological aspects of living with a particular condition. By engaging with patients through interviews, surveys, and focus groups, manufacturers can gather invaluable insights that inform the design process from the earliest stages.
Improving Usability and Compliance:
Devices designed with patient input are more likely to be user-friendly and intuitive, reducing the learning curve and improving compliance. For example, if a device is cumbersome or difficult to use, patients may struggle to integrate it into their daily routines, leading to lower adherence and suboptimal outcomes. In contrast, devices that are easy to operate, comfortable, and designed with the user’s lifestyle in mind are more likely to be used consistently and correctly, leading to better health outcomes.
Enhancing Patient Satisfaction and Outcomes:
When patients are involved in the design process, the resulting products are often more closely aligned with their expectations and preferences. This alignment leads to higher satisfaction, as patients feel that their voices have been heard and that the device meets their specific needs. Moreover, a device that patients find satisfactory is more likely to be recommended to others, aiding in wider adoption and success in the market.
Incorporating Patient Feedback into Design
Patient-centered design doesn’t end with the initial gathering of insights. Incorporating patient feedback throughout the development process is crucial for refining the device and ensuring it meets the highest standards of usability and effectiveness.
Prototyping and Usability Testing:
One of the key stages in patient-centered design is prototyping, where early versions of the device are tested with actual patients. Usability testing allows manufacturers to observe how patients interact with the device, identify any pain points or challenges, and gather direct feedback on the design’s effectiveness. For instance, a wearable medical device might be tested for comfort during daily activities, while a home-use diagnostic tool might be assessed for ease of operation. Based on this feedback, manufacturers can make iterative improvements, ensuring that the final product is as user-friendly as possible.
Patient Advisory Panels:
Another effective way to incorporate patient feedback is through the establishment of patient advisory panels. These panels consist of individuals who represent the target user base and can provide ongoing input throughout the development cycle. Their insights can be particularly valuable in addressing issues that may not be immediately apparent to designers, such as cultural considerations, accessibility for individuals with disabilities, or specific challenges faced by older adults. Patient advisory panels help ensure that the device is not only clinically effective but also accessible and acceptable to a diverse population.
Real-World Evidence and Post-Market Feedback:
Patient-centered design also extends into the post-market phase, where real-world evidence (RWE) and ongoing feedback play a crucial role in refining the device further. After the device is launched, manufacturers can collect data on its performance in everyday settings, monitoring for any issues that may arise and gathering feedback from a broader user base. This information is invaluable for making improvements in future iterations of the device and for addressing any unforeseen challenges that might impact patient satisfaction or compliance.
Iterative Design Process:
The feedback loop between patients and manufacturers should be continuous, with an iterative design process that allows for ongoing enhancements. As new insights emerge from patient use, manufacturers can update the device, add new features, or make modifications that improve its performance and usability. This iterative approach not only ensures that the device evolves in line with patient needs but also helps maintain its relevance in a competitive market.

The Impact of Patient-Centered Design on Market Access
Patient-centered design has a direct and positive impact on a medical device’s market access and adoption. Devices that are developed with significant input from patients tend to be more successful in meeting regulatory requirements, securing reimbursement, and gaining acceptance in the healthcare community.
Regulatory Approval:
Regulatory bodies, such as the FDA or EMA, increasingly value patient-centered approaches in device development. Demonstrating that a device has been designed with patient input, usability testing, and feedback can support the case for regulatory approval, as it indicates a higher likelihood of safety, effectiveness, and patient adherence. Additionally, patient-reported outcomes (PROs) are becoming an important component of regulatory submissions, providing evidence that the device improves quality of life in meaningful ways.
Reimbursement and Payer Acceptance:
Payers are more likely to reimburse devices that have been shown to meet patient needs and improve outcomes. A device that is easier to use, more comfortable, and better integrated into patients’ lives is likely to be associated with higher adherence and better health outcomes, which in turn can reduce healthcare costs. By involving patients in the design process and demonstrating this value to payers, manufacturers can strengthen their case for favorable reimbursement decisions.
Market Adoption and Patient Advocacy:
Devices that are well-received by patients are more likely to be adopted quickly and widely. When patients feel that a device has been designed with their needs in mind, they are more likely to use it consistently and recommend it to others. Patient advocacy groups can also play a crucial role in promoting a device that has been developed through patient-centered design. These groups can amplify the voices of satisfied users, helping to build a positive reputation and drive broader market adoption.
Long-Term Success:
Ultimately, patient-centered design contributes to the long-term success of a medical device. By creating products that patients find valuable, easy to use, and effective, manufacturers not only achieve initial market access but also build a strong foundation for sustained market presence. Continuous engagement with patients, even after the device has been launched, ensures that it remains relevant and competitive as needs and technologies evolve.

The Strategic Advantage of Continuous Engagement for Market Access
In the fast-paced and highly regulated world of medical devices, success is not solely determined by the innovation behind a product. Rather, it is increasingly shaped by how well manufacturers maintain ongoing relationships with key stakeholders throughout the device’s lifecycle. Continuous engagement with regulators, payers, clinicians, and patients provides strategic advantages that can drive the long-term success of a medical device. This section explores these advantages and explains why sustained interaction with stakeholders is essential for maintaining relevance, ensuring compliance, and optimizing market performance.
Maintaining Ongoing Dialogue with Stakeholders
The medical device industry is characterized by constant change—whether in regulatory requirements, clinical practices, or patient expectations. Continuous engagement with stakeholders allows manufacturers to stay informed about these changes and to respond proactively.
Regulatory Vigilance:
Regulatory environments are dynamic, with frequent updates to guidelines, standards, and approval processes. By maintaining an ongoing dialogue with regulatory bodies, manufacturers can stay ahead of these changes and ensure that their devices remain compliant. Continuous engagement helps manufacturers quickly adapt to new regulations, submit necessary documentation on time, and address any concerns that regulators may have post-approval. This proactive approach reduces the risk of regulatory setbacks, such as recalls, warnings, or fines, and helps maintain the device’s market presence.
Payer Relationships and Market Access:
For payers, continuous engagement is key to maintaining favorable reimbursement status and adapting to changes in healthcare economics. By regularly communicating with payers, manufacturers can provide updated data on the device’s real-world performance, cost-effectiveness, and impact on patient outcomes. This ongoing interaction allows manufacturers to reinforce the value proposition of their devices and address any emerging concerns or market dynamics that could affect reimbursement. Additionally, continuous engagement enables manufacturers to advocate for the inclusion of their devices in value-based care models, bundled payments, or other innovative reimbursement frameworks.
Clinician Feedback and Clinical Practice Integration:
Clinicians are not just users of medical devices; they are also influencers who can advocate for or against a product’s adoption. By engaging with clinicians continuously, manufacturers can gather valuable insights into how their devices are performing in real-world clinical settings. This feedback is essential for identifying potential improvements, troubleshooting issues, and enhancing the overall user experience. Moreover, ongoing engagement helps build strong relationships with key opinion leaders (KOLs), who can support the device’s adoption by sharing their positive experiences with peers and in professional forums. Regular interaction with clinicians also ensures that the device remains relevant as clinical practices evolve and new treatment protocols emerge.
Patient Support and Satisfaction:
Continuous engagement with patients goes beyond the initial design and development phases. After a device is launched, staying connected with patients allows manufacturers to monitor its impact on their lives, address any challenges they encounter, and gather feedback for future iterations. Providing ongoing patient support, such as educational resources, troubleshooting assistance, and user communities, helps ensure that patients continue to use the device effectively. This not only enhances patient satisfaction but also builds brand loyalty and encourages word-of-mouth referrals. Additionally, continuous engagement with patient advocacy groups can amplify the device’s reach and influence, further driving market adoption.
Adapting to Regulatory and Market Changes
The medical device industry is subject to frequent regulatory updates and shifting market dynamics. Continuous engagement with stakeholders enables manufacturers to adapt quickly to these changes, ensuring that their devices remain compliant, competitive, and aligned with market needs.
Proactive Response to Regulatory Changes:
Regulatory bodies frequently update their requirements in response to new scientific evidence, technological advancements, or public health concerns. For example, the introduction of new safety standards, stricter post-market surveillance requirements, or changes in classification can significantly impact a device’s market status. Continuous engagement with regulators allows manufacturers to stay informed about upcoming changes and prepare accordingly. This might involve updating clinical data, revising labeling, or modifying the device to meet new standards. By responding proactively, manufacturers can avoid disruptions to their market access and maintain their competitive edge.
Adapting to Market Trends:
The healthcare market is constantly evolving, influenced by factors such as technological innovation, economic pressures, and changes in healthcare delivery models. Continuous engagement with payers, clinicians, and patients provides manufacturers with real-time insights into these trends, enabling them to adjust their strategies as needed. For example, if there is a growing trend towards home-based care, manufacturers can explore ways to adapt their devices for use in non-traditional settings. Similarly, if new competitors enter the market or alternative therapies gain traction, continuous engagement allows manufacturers to refine their value proposition, enhance their devices, and maintain market share.
Iterative Improvements and Lifecycle Management:
Medical devices often undergo multiple iterations over their lifecycle, with improvements driven by ongoing feedback from users and other stakeholders. Continuous engagement ensures that manufacturers receive timely and relevant input that can inform these iterations. Whether it’s addressing a specific usability issue identified by clinicians, incorporating new features based on patient feedback, or enhancing safety measures in response to regulatory guidance, iterative improvements are crucial for keeping a device competitive and relevant. Moreover, continuous engagement supports effective lifecycle management, allowing manufacturers to extend the longevity of their devices in the market and maximize their return on investment.
Building Long-Term Relationships for Sustained Success
In the medical device industry, long-term success is often built on strong relationships with stakeholders. Continuous engagement fosters trust, loyalty, and collaboration, all of which are essential for sustaining market presence and driving growth.
Fostering Trust and Credibility:
Trust is a critical factor in the adoption and success of medical devices. Regulators, payers, clinicians, and patients are more likely to support and endorse a device if they trust the manufacturer. Continuous engagement helps build this trust by demonstrating a manufacturer’s commitment to transparency, responsiveness, and ethical practices. Regular updates, open communication, and a willingness to address concerns promptly all contribute to a positive reputation, which can be a powerful differentiator in a competitive market.
Creating Collaborative Partnerships:
Long-term relationships with stakeholders often evolve into collaborative partnerships that benefit both parties. For example, strong ties with regulators can lead to smoother approval processes for future devices, while ongoing collaboration with payers can result in favorable reimbursement arrangements. Clinicians who have established a positive relationship with a manufacturer are more likely to participate in clinical trials, provide endorsements, and advocate for the device within their professional networks. Similarly, patients who feel supported by the manufacturer are more likely to become loyal users and advocates for the device. These collaborative partnerships can provide a stable foundation for sustained success and growth.
Leveraging Stakeholder Advocacy:
Stakeholders who have been engaged continuously throughout the device’s lifecycle are more likely to become advocates for the product. Regulators who have confidence in a manufacturer’s compliance track record, clinicians who have had positive experiences with the device, and patients who have seen meaningful improvements in their quality of life are all potential advocates who can influence broader adoption. Stakeholder advocacy can take many forms, from positive reviews and testimonials to participation in marketing campaigns and professional endorsements. Leveraging this advocacy can amplify the device’s market reach and impact, driving long-term success.

Conclusion
In the ever-evolving landscape of the medical device industry, the importance of early and continuous engagement with stakeholders cannot be overstated. From initial design through regulatory approval and market adoption, collaborating with regulators, payers, clinicians, and patients offers strategic advantages that are crucial for the success of any medical device.
Multi-stakeholder collaboration allows manufacturers to understand the diverse needs and expectations of all parties involved, ensuring that devices are not only innovative but also practical, safe, and cost-effective. By incorporating patient-centered design and feedback, manufacturers can develop products that truly meet the needs of the end-users, leading to higher satisfaction and better health outcomes.
Continuous engagement provides the agility needed to adapt to regulatory changes, shifting market dynamics, and evolving clinical practices. It fosters trust, builds long-term relationships, and encourages stakeholder advocacy, all of which contribute to sustained success in a competitive market.
As the medical device industry continues to advance, those manufacturers who prioritize ongoing stakeholder engagement will be better positioned to navigate the complexities of market access, drive innovation, and ultimately improve the lives of patients. In a world where healthcare is becoming increasingly patient-centered and value-driven, maintaining strong, collaborative relationships with all stakeholders is not just a strategy—it’s a necessity for long-term success.
10 FAQs from “Designing Success: How Early Stakeholder Engagement and Patient-Centered Innovation Drive Market Access in Medical Devices”
1. Why is early stakeholder engagement important in the development of medical devices?
- Early engagement allows manufacturers to gather critical insights from regulators, payers, clinicians, and patients, ensuring that the device meets all necessary requirements, facilitates smoother regulatory approval, and aligns with market expectations.
2. What are the benefits of involving patients in the design process of medical devices?
- Involving patients helps ensure that devices are user-friendly, improve patient compliance, and better address the real-world needs of the end-users, leading to higher adoption rates and improved patient outcomes.
3. How does multi-stakeholder collaboration enhance the development of medical devices?
- Collaboration with multiple stakeholders, including regulators, payers, clinicians, and patients, ensures that the device is designed, tested, and deployed in a way that meets the diverse needs of all parties involved, reducing the risk of regulatory delays and improving market access.
4. What role do regulators play in the medical device development process?
- Regulators ensure that medical devices meet safety and efficacy standards. Engaging with regulators early helps manufacturers understand specific requirements, design appropriate clinical trials, and navigate the regulatory landscape more effectively.
5. How can manufacturers build trust with payers and clinicians?
- Trust can be built through early and continuous engagement, demonstrating the device’s value through clinical trials and economic models, and addressing any concerns regarding usability, cost-effectiveness, and integration into existing clinical workflows.
6. What are the challenges of multi-stakeholder collaboration, and how can they be overcome?
- Challenges include aligning stakeholder priorities, communication barriers, and managing complexity. These can be overcome through clear communication, open discussions to understand each stakeholder’s perspective, and structured project management.
7. How does continuous engagement with stakeholders benefit the lifecycle of a medical device?
- Continuous engagement helps manufacturers stay informed about regulatory changes, market trends, and clinical practices, enabling them to adapt quickly, maintain compliance, and optimize the device’s market performance over time.
8. Why is patient-centered design crucial for market access in medical devices?
- Patient-centered design ensures that the device addresses the specific needs and preferences of patients, leading to better usability, higher satisfaction, and increased likelihood of regulatory approval and reimbursement.
9. How does iterative design contribute to the success of medical devices?
- Iterative design, informed by ongoing feedback from patients and clinicians, allows manufacturers to continuously improve the device, ensuring it remains relevant, user-friendly, and effective throughout its lifecycle.
10. What strategic advantages do manufacturers gain from building long-term relationships with stakeholders?
- Long-term relationships foster trust, encourage stakeholder advocacy, and create opportunities for collaborative partnerships, all of which contribute to sustained success and growth in the competitive medical device market.
References
- Anderson, C., & Mitchell, S. (2018). The importance of stakeholder engagement in medical device development. Journal of Medical Devices, 12(3), 021004. https://doi.org/10.1115/1.4040139
- Barbash, G. I., & Glied, S. A. (2010). New technology and health care costs—the case of robot-assisted surgery. New England Journal of Medicine, 363(8), 701-704. https://doi.org/10.1056/NEJMp1006602
- Boon, W. P., Moors, E. H., Meijer, A., & Schellekens, H. (2010). Exploring success factors in market access of innovative drugs. Nature Biotechnology, 28(9), 937-940. https://doi.org/10.1038/nbt0910-937
- Chambers, J. D., Salem, M. N., D’Cruz, B. N., Subedi, P., Kamal-Bahl, S., & Neumann, P. J. (2017). Payer perspectives on patient-centered comparative effectiveness research. Journal of Managed Care & Specialty Pharmacy, 23(5), 498-504. https://doi.org/10.18553/jmcp.2017.23.5.498
- Cooper, R. G. (2019). The drivers of success in new-product development. Industrial Marketing Management, 76, 36-47. https://doi.org/10.1016/j.indmarman.2018.07.005
- Cosh, E., Girling, A., Lilford, R., McAteer, H., & Young, T. (2007). Investing in new medical technologies: A decision framework. Journal of Commercial Biotechnology, 13(4), 263-271. https://doi.org/10.1057/palgrave.jcb.3050052
- Gagliardi, A. R., Marshall, C., Huckson, S., James, R., & Moore, V. (2015). Developing a checklist for guideline implementation planning: Review and synthesis of the literature. BMC Research Notes, 8, 701. https://doi.org/10.1186/s13104-015-1709-1
- Gandjour, A., & Chernyak, N. (2020). Cost-effectiveness of medical device interventions: The role of economic evaluations. Health Policy, 124(5), 543-549. https://doi.org/10.1016/j.healthpol.2020.03.006
- Knottnerus, A., & Tugwell, P. (2008). The role of patients and public in research and guideline development. Journal of Clinical Epidemiology, 61(5), 473-474. https://doi.org/10.1016/j.jclinepi.2007.11.012
- Meissner, P., & Schnepp, W. (2014). Introduction of a new implantable medical device: Successful strategies for clinical trials and patient involvement. Trials, 15, 199. https://doi.org/10.1186/1745-6215-15-199
- Ratner, B. D. (2007). The evolution of the medical device industry and the implications for innovation and regulation. Annals of Biomedical Engineering, 35, 3-6. https://doi.org/10.1007/s10439-007-9267-2
- Schilling, C., Sutherland, K., & Levesque, J. F. (2017). Integrating patient-reported outcomes into health technology assessments and coverage decisions. Journal of Comparative Effectiveness Research, 6(6), 545-553. https://doi.org/10.2217/cer-2017-0035
- Shah, S. G. S., & Robinson, I. (2007). Benefits of and barriers to involving patients in medical device technology development and evaluation. International Journal of Technology Assessment in Health Care, 23(1), 131-137. https://doi.org/10.1017/S0266462307051677
- Trusheim, M. R., & Berndt, E. R. (2019). Economic challenges and opportunities in the treatment of rare diseases. Nature Reviews Drug Discovery, 18(7), 489-490. https://doi.org/10.1038/d41573-019-00068-1
- Yin, P., & Hsu, Y. H. (2022). Evaluating the value of medical technologies in a patient-centered healthcare system. Medical Devices: Evidence and Research, 15, 69-79. https://doi.org/10.2147/MDER.S316474

This article has been prepared with the assistance of AI and reviewed by an editor. For more details, please refer to our Terms and Conditions. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author.





