10 key takeaways from the article “Securing Access for Gene Therapies”:
- Revolutionary Potential: Gene therapies offer a groundbreaking approach to treating rare and genetic diseases by addressing the root cause, rather than just managing symptoms.
- High Costs: The complex development, production, and delivery of gene therapies result in extremely high costs, often reaching millions of dollars per patient, creating financial challenges for healthcare systems.
- Regulatory Complexities: Regulatory approval processes for gene therapies are more stringent and time-consuming due to the need for long-term data and safety monitoring, delaying patient access.
- Uncertainty of Long-Term Effects: Gene therapies alter the genetic code, raising concerns about unknown long-term effects, and necessitating continuous monitoring and real-world data collection.
- Manufacturing Challenges: The production of gene therapies requires specialized infrastructure, highly skilled personnel, and advanced technology, making scaling up and global distribution difficult.
- Innovative Payment Models: Outcome-based agreements and annuity-based payments are being developed to ease the financial burden on healthcare systems and align costs with therapeutic success.
- Regulatory Reforms: Accelerated approval pathways, such as those by the FDA and EMA, are crucial for speeding up the availability of gene therapies, particularly for patients with no other treatment options.
- Public-Private Partnerships: Collaborative efforts between governments, private companies, and NGOs can reduce costs, improve access, and promote infrastructure development, especially in low- and middle-income countries.
- Patient Support Programs: Manufacturers are implementing programs to assist patients financially and logistically, ensuring broader access to these high-cost treatments.
- Future Innovations: Advances in gene-editing technologies and manufacturing efficiency are expected to lower costs, enhance precision, and make gene therapies more accessible globally, particularly in developing regions.
Gene therapies represent a groundbreaking frontier in the treatment of rare and previously incurable diseases. By targeting the genetic root causes of these conditions, gene therapies hold the promise of not just managing symptoms but offering long-lasting, potentially curative solutions. However, the complex nature of these therapies, combined with their high cost and intricate regulatory pathways, poses significant challenges in securing patient access.
I will deep dive into this article to explore the key challenges and strategies for ensuring broad and equitable access to gene therapies while considering future developments in this evolving field.
The Evolution of Gene Therapies
The concept of gene therapy first emerged decades ago, with the idea that by altering a patient’s DNA, medical professionals could correct faulty genes responsible for debilitating or fatal diseases. However, it wasn’t until recently that advances in molecular biology, gene editing technologies (such as CRISPR-Cas9), and viral vector delivery systems brought this vision closer to reality. Today, gene therapies have achieved regulatory approval for several conditions, particularly rare diseases like spinal muscular atrophy (SMA) and inherited retinal dystrophy.
Gene therapies work by delivering a functional copy of a gene into a patient’s cells, either by using viral vectors or non-viral techniques like nanoparticles. The ultimate goal is to restore normal function at the cellular level, correcting the genetic defects that cause disease. This differs fundamentally from traditional treatments, which often focus on managing symptoms or slowing disease progression. By addressing the root cause, gene therapies offer the potential for durable, long-term, or even curative outcomes in a single administration.
Expanding Therapeutic Potential
While the initial focus of gene therapies has been on rare genetic disorders, the therapeutic potential extends far beyond. Research is underway to explore their applicability in more prevalent diseases such as cancer, cardiovascular diseases, and neurodegenerative conditions like Parkinson’s and Alzheimer’s. For instance, CAR-T cell therapies, a form of gene therapy, have shown remarkable success in treating certain blood cancers, marking the beginning of gene therapies’ expansion into broader therapeutic areas.
Furthermore, advancements in gene-editing technologies such as CRISPR, base editing, and prime editing hold the potential to develop more precise and targeted gene therapies. These next-generation therapies may have fewer off-target effects and greater safety profiles, which could accelerate the adoption of gene therapies in mainstream medicine.
However, as the scope of gene therapies grows, so too does the complexity of ensuring timely access to patients who could benefit from these innovations. Addressing the underlying challenges, particularly in terms of cost, regulatory frameworks, and equitable access, is critical to the future of gene therapies.
Challenges in Securing Access to Gene Therapies
Securing access to gene therapies presents a multifaceted challenge for healthcare systems worldwide. These revolutionary treatments, while offering life-changing potential for patients with rare genetic disorders, come with significant barriers that hinder widespread availability.
We can define the main challenges in securing access to gene therapies as;
- High Cost and Economic Burden
- Regulatory and Reimbursement Pathways
- Manufacturing Complexities
- Uncertainty of Long-Term Effects
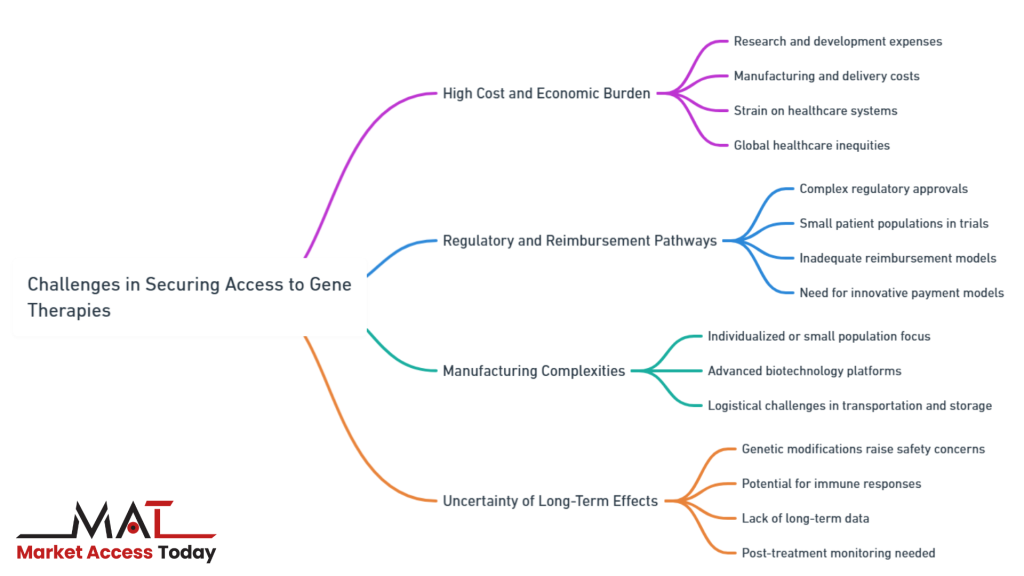
Among the most critical challenges are the high costs associated with research, development, and delivery, complex regulatory and reimbursement frameworks, and intricate manufacturing processes. As healthcare systems grapple with these issues, the need for innovative solutions becomes increasingly urgent to ensure that patients, regardless of their geographic or economic standing, can access these groundbreaking therapies.
Let`s deep dive into the main challenges in securing access to gene therapies.
a. High Cost and Economic Burden
Gene therapies often come with unprecedented costs, primarily due to the highly complex research and development processes, manufacturing, and delivery mechanisms involved. These treatments can range from several hundred thousand to millions of dollars per patient. For example, Zolgensma, a gene therapy used to treat spinal muscular atrophy (SMA), costs over $2 million for a single dose, making it one of the most expensive drugs ever marketed.
The high upfront costs pose significant challenges to healthcare systems, payers, and governments. Even in high-income countries, ensuring that gene therapies are available to all eligible patients can strain national healthcare budgets. For middle- and low-income countries, the financial burden can be even greater, raising concerns about healthcare inequities and the potential for gene therapies to exacerbate global disparities in access to innovative treatments.
b. Regulatory and Reimbursement Pathways
The regulatory approval process for gene therapies is more complex than for conventional drugs. Regulatory agencies such as the U.S. FDA and the European Medicines Agency (EMA) require gene therapies to undergo stringent evaluations to ensure safety, efficacy, and durability of effects. Since many gene therapies are designed for rare diseases, clinical trials often involve small patient populations, which can create challenges in meeting the statistical rigor required for approval.
Moreover, existing reimbursement systems are not well-suited to cover the high one-time costs associated with gene therapies. Traditional payment models, which spread costs over time as patients use chronic treatments, may not align with the curative potential of gene therapies. As a result, payers and manufacturers must explore new pricing and reimbursement models, such as value-based agreements, to balance costs with the benefits these therapies bring to patients and healthcare systems.
c. Manufacturing Complexities
Gene therapies are often tailored to individual patients or very small patient populations, which makes scaling up manufacturing a formidable challenge. The production process requires advanced biotechnological platforms, highly skilled labor, and stringent quality controls to ensure the consistency and safety of the therapies. Manufacturing facilities must be equipped to handle live viral vectors or complex gene-editing technologies, which can be both time-consuming and expensive.
Additionally, transporting and storing gene therapies often requires specialized logistics, including ultra-cold storage for certain products. These logistical hurdles can limit the ability to distribute gene therapies to a global patient base, especially in regions with less developed healthcare infrastructure.
d. Uncertainty of Long-Term Effects
Gene therapies introduce profound changes at the genetic level, which presents a unique challenge in terms of uncertainty about their long-term effects. Unlike traditional treatments that manage symptoms without altering the patient’s DNA, gene therapies work by modifying the genetic code itself, raising concerns about the potential for unintended consequences. While these therapies offer promising results in the short term, the lack of extensive long-term data means that the full spectrum of their effects is not yet fully understood.
For example, gene therapy might trigger immune responses, lead to off-target gene modifications, or create new mutations that could cause complications years after the initial treatment. This uncertainty makes regulatory approval processes more complex and creates a need for post-treatment monitoring to ensure that patients remain healthy in the long run. Furthermore, payers and healthcare providers may hesitate to fully embrace these therapies without a clearer understanding of the potential risks, making it difficult to establish confidence in their widespread use.
The challenge of uncertainty underscores the importance of ongoing research, patient follow-up, and the development of new frameworks to evaluate the long-term safety and efficacy of gene therapies. Balancing the promise of a potential cure with the risks of altering the human genome is crucial in building trust among patients, healthcare professionals, and regulatory bodies.
Strategies to Ensure Access to Gene Therapies
Addressing the complex challenges of securing access to gene therapies requires innovative and multifaceted strategies. Stakeholders, including governments, healthcare providers, regulatory bodies, and pharmaceutical companies, are exploring a range of solutions to ensure that these life-changing treatments are available to patients who need them.
We can define the main strategies to ensure access to gene therapies as;
- Innovative Payment and Pricing Models
- Regulatory Reforms
- Public-Private Partnerships
- Patient Support Programs
- Generate More Real-World Data
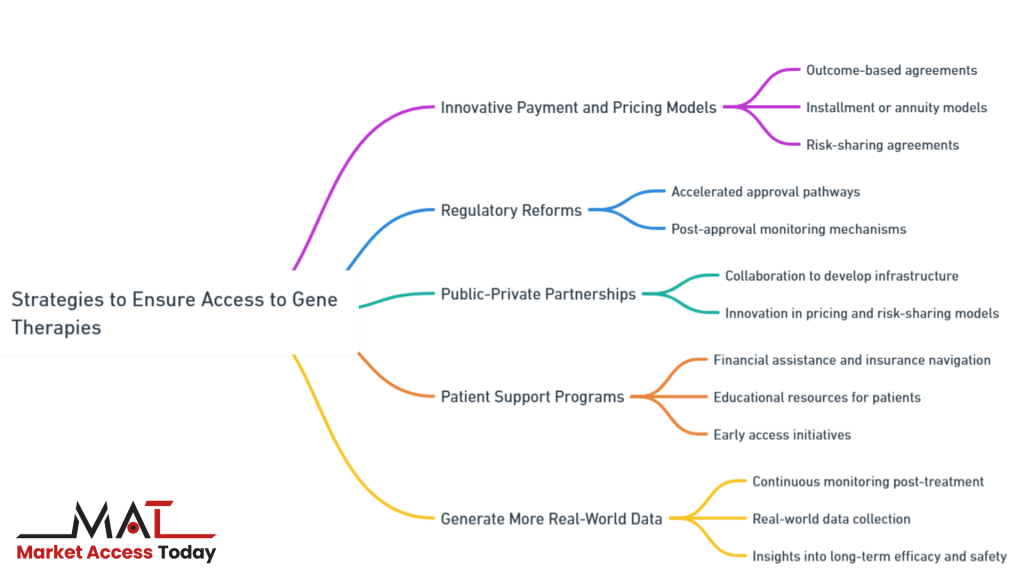
These strategies aim to overcome the financial, regulatory, and logistical barriers that hinder access while also promoting global equity in the availability of gene therapies. By implementing new payment models, reforming regulatory pathways, fostering public-private partnerships, and offering patient support programs, stakeholders can mitigate the challenges and help secure access to gene therapies for a broader population.
Let`s deep dive into the main strategies to ensure access to gene therapies.
a. Innovative Payment and Pricing Models
New payment models are critical to ensure that gene therapies are accessible without overwhelming healthcare systems. Outcome-based agreements, also known as “pay-for-performance” models, link payments to the real-world effectiveness of the therapy. For example, if a patient’s health improves or if the treatment meets specific milestones over time, the payer provides payment to the manufacturer. These models help mitigate financial risk for healthcare systems by ensuring that payments are only made when the therapy delivers on its promise.
Other solutions, such as installment payments or annuity-based models, spread the cost of gene therapies over several years, making the financial impact more manageable. Manufacturers are also exploring models that involve risk-sharing agreements, in which both payers and manufacturers assume responsibility for the long-term success of the therapy.
b. Regulatory Reforms
Regulatory agencies are taking steps to adapt their frameworks to accommodate gene therapies. Accelerated approval pathways, such as the FDA’s Breakthrough Therapy Designation and the EMA’s PRIME scheme, allow promising gene therapies to reach patients faster by expediting the review process. These programs are designed to facilitate earlier access to life-saving treatments, especially for patients with no alternative options.
Regulators are also working on post-approval monitoring mechanisms to assess the long-term safety and efficacy of gene therapies, given the inherent challenges in collecting long-term data during clinical trials. This ensures that even after market approval, gene therapies are continuously evaluated, which helps build trust in their use.
c. Public-Private Partnerships
Public-private partnerships can play a pivotal role in overcoming the barriers to access. Governments, regulatory bodies, non-governmental organizations (NGOs), and industry stakeholders can collaborate to develop shared infrastructure, subsidize costs, and establish policies that promote equitable access. For example, partnerships could help establish manufacturing hubs in low- and middle-income countries, ensuring a more equitable distribution of gene therapies globally. These collaborations can also facilitate the sharing of resources, such as clinical trial data, expertise, and regulatory best practices, which can accelerate the development and approval process for gene therapies across different regions.
Moreover, public-private partnerships can drive innovation in pricing models and risk-sharing agreements. By pooling resources and expertise, governments and private entities can co-create mechanisms to absorb the financial risks associated with gene therapies, thus reducing the burden on public healthcare systems. This collaborative approach can ensure that gene therapies are not confined to wealthy nations but are accessible to a broader patient base, including those in underfunded healthcare systems. Additionally, partnerships between governments and industry players can help build the necessary healthcare infrastructure, such as advanced labs and trained personnel, that is essential for the safe and effective delivery of these complex therapies.
d. Patient Support Programs
Manufacturers are increasingly offering patient support programs that provide financial assistance, insurance navigation, and logistical support. These programs are designed to help patients overcome the financial and practical hurdles associated with gene therapies. By providing resources to patients and families, these programs can alleviate some of the economic strain and make it easier for patients to access the therapies they need. In many cases, support programs also offer educational services, ensuring that patients and caregivers understand the treatment, the potential risks, and how to manage follow-up care effectively.
Additionally, patient support programs may work in collaboration with healthcare providers and advocacy groups to streamline access. These partnerships can assist patients in navigating the complex insurance and reimbursement processes, ensuring that financial barriers do not prevent patients from receiving the life-saving treatments they require. Some manufacturers are even establishing compassionate use programs or early access initiatives, allowing patients in critical need to access gene therapies before they receive full regulatory approval. This direct assistance can significantly improve access and outcomes for patients, particularly in regions where healthcare coverage is limited or insufficient to cover the costs of advanced therapies.
e. Generate More Real-World Data
Given the uncertainty surrounding the long-term effects of gene therapies, generating more real-world data (RWD) is essential for understanding their full impact on patients over time. Clinical trials for gene therapies often involve small, highly controlled patient populations, making it difficult to predict how these therapies will perform in broader, more diverse populations. Real-world data collection allows for the continuous monitoring of patients after treatment, offering insights into both the efficacy and safety of gene therapies outside the confines of a clinical trial. This data can help fill gaps in knowledge about potential side effects, long-term durability, and the overall risk-benefit profile of these therapies.
Regulatory bodies, healthcare providers, and manufacturers can collaborate to establish frameworks for RWD generation, using patient registries, electronic health records, and post-market surveillance programs. By tracking outcomes over time, these efforts can reduce uncertainty and build confidence in gene therapies, reassuring payers, patients, and healthcare professionals. Additionally, real-world data can inform future research and development, guide pricing models based on long-term effectiveness, and help regulatory bodies refine their approval processes. In the long run, collecting and analyzing RWD will be crucial to mitigating the risks associated with altering patients’ genetic material and ensuring that gene therapies deliver on their promise of curative, life-changing treatments.
The Future of Gene Therapy Access
As gene therapies continue to advance and become more prevalent in the treatment of a wide range of diseases, ensuring equitable access is increasingly important. While these innovative treatments offer tremendous potential, there are numerous challenges that need to be addressed to make gene therapies accessible to all who need them, regardless of geographic or economic barriers.
We can discuss the future of gene therapy access under the titles;
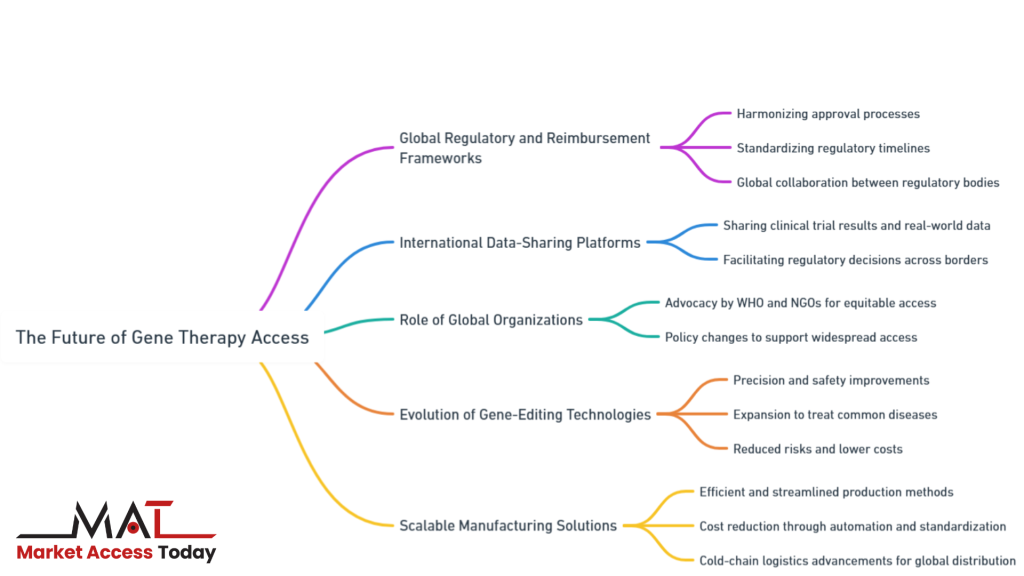
- Global Regulatory and Reimbursement Frameworks
- International Data-Sharing Platforms
- Role of Global Organizations
- Evolution of Gene-Editing Technologies
- Scalable Manufacturing Solutions
By exploring these aspects in greater depth, we can understand the necessary steps to overcome existing obstacles and pave the way for widespread availability of gene therapies.
Let`s deep dive into these.
Global Regulatory and Reimbursement Frameworks
Future developments may include the creation of Global Regulatory and Reimbursement Frameworks aimed at harmonizing approval processes across countries and regions. Currently, disparities exist between nations in terms of approval timelines and regulatory stringency, which can result in delays or unequal access to therapies in different regions. Global collaboration between regulatory bodies could help standardize these processes, allowing for faster approvals, particularly in low- and middle-income countries, where healthcare infrastructure may not be equipped to handle the high complexity of gene therapies.
International Data-Sharing Platforms
One approach might involve leveraging International Data-Sharing Platforms that facilitate the exchange of clinical trial results, real-world data, and regulatory decisions across borders. By creating a more unified global framework, regions with fewer resources could benefit from the research and regulatory advancements made elsewhere, potentially reducing the time it takes for gene therapies to become accessible in emerging markets.
Role of Global Organizations
Additionally, Global Organizations, such as the World Health Organization (WHO) and global NGOs, will play a critical role in advocating for policy changes that support fair and widespread access to these life-changing treatments. These organizations can help push for global policy changes that ensure equitable access to gene therapies, particularly in regions where healthcare coverage is limited.
Evolution of Gene-Editing Technologies
The continued evolution of Gene-Editing Technologies, such as CRISPR and base-editing, will likely make gene therapies more precise, safer, and cost-effective. These innovations will reduce risks related to off-target effects and unwanted mutations, addressing concerns about long-term safety. As these tools become more refined, they may open up new therapeutic possibilities, treating not only rare genetic disorders but also common diseases, such as cancer, diabetes, and cardiovascular diseases. This expansion into broader indications will increase the demand for gene therapies, necessitating scalable solutions to ensure that healthcare systems can meet the growing need.
Furthermore, advancements in gene-editing technologies could help lower the barriers to entry for developers and healthcare systems, making gene therapies more widely accessible. With improvements in delivery mechanisms, such as non-viral vectors and in vivo editing techniques, the manufacturing and delivery processes could become less expensive and more streamlined. As production methods become more efficient and scalable, the overall cost of gene therapies is expected to decrease, allowing for broader access across different socioeconomic strata and regions. Additionally, simplified production techniques could make it feasible for more countries to develop their own gene therapy manufacturing capabilities, reducing reliance on a few specialized centers.
Scalable Manufacturing Solutions
In the long term, as more gene therapies gain approval and the technologies mature, Scalable Manufacturing Solutions are expected to become more efficient. This could reduce production costs, making gene therapies more affordable on a global scale. Automation in biomanufacturing, along with the development of standardized production platforms, could allow for larger-scale production without compromising the safety and quality of the therapies.
Improvements in Cold-Chain Logistics and Storage Technology could ensure that gene therapies are distributed to more remote regions, overcoming some of the existing logistical barriers.
In conclusion, the future of gene therapy access will depend on a combination of global collaboration, technological innovation, and policy reform. With the right infrastructure and strategies in place, gene therapies have the potential to reach a much larger patient population, transforming healthcare and improving the lives of millions worldwide. However, achieving this vision requires concerted efforts to address cost, regulatory, and logistical barriers, ensuring that the promise of gene therapies is realized equitably across the globe.
Conclusion
Gene therapies represent a revolutionary approach to treating some of the most challenging genetic disorders. However, the high costs, complex regulatory requirements, and logistical challenges associated with these treatments present significant barriers to patient access.
By adopting innovative pricing models, streamlining regulatory processes, fostering public-private partnerships, and supporting patients directly, stakeholders can work together to secure broad and equitable access to these life-changing therapies. As the field continues to evolve, the goal of providing curative treatments to all patients who need them is within reach, paving the way for a future where genetic diseases no longer pose insurmountable obstacles to health and well-being.
Guvenc Kockaya, September 2024
10 FAQs about securing access to gene therapies:
- What are gene therapies? Gene therapies are medical treatments that involve altering a patient’s genetic material to treat or prevent diseases, often targeting the root cause of genetic disorders rather than just managing symptoms.
- What makes gene therapies so expensive? Gene therapies are costly due to their complex research and development, manufacturing processes, and the personalized nature of many treatments, often costing millions of dollars per patient.
- Why is there uncertainty around gene therapies? Since gene therapies change the patient’s genetic code, there are concerns about unknown long-term effects. The lack of extensive long-term data makes it difficult to predict outcomes over many years.
- How are gene therapies regulated? Regulatory agencies like the FDA and EMA have strict guidelines for approving gene therapies, including safety, efficacy, and durability assessments. Accelerated approval pathways are sometimes used to speed up access for life-threatening conditions.
- How can gene therapy access be made more affordable? Innovative payment models like outcome-based agreements and installment payments are being developed to align the high upfront costs with the long-term benefits of gene therapies, making them more manageable for healthcare systems.
- What role do public-private partnerships play in gene therapy access? Public-private partnerships can help subsidize costs, develop manufacturing infrastructure, and implement global policies to ensure equitable access to gene therapies, particularly in low-income regions.
- Why is real-world data important for gene therapies? Real-world data provides insights into the long-term effects and safety of gene therapies outside of controlled clinical trials, helping to reduce uncertainty and improve treatment strategies.
- How can patients get financial help for gene therapies? Many manufacturers offer patient support programs, which include financial assistance, insurance navigation, and logistical support to help patients access gene therapies.
- Will gene therapies become more widely available in the future? As gene-editing technologies improve and manufacturing processes become more efficient, the costs of gene therapies are expected to decrease, making them more accessible globally.
- How do regulatory reforms help improve access to gene therapies? Regulatory reforms, including accelerated approval pathways and harmonization across regions, can speed up the process of bringing gene therapies to market, ensuring patients in different parts of the world can access them sooner.
References
- Maeder, M. L., & Gersbach, C. A. (2016). Genome-editing technologies for gene and cell therapy. Molecular Therapy, 24(3), 430–446. https://doi.org/10.1038/mt.2016.10
- High, K. A., & Roncarolo, M. G. (2019). Gene therapy. New England Journal of Medicine, 381(5), 455–464. https://doi.org/10.1056/NEJMra1706910
- Al-Zaidy, S. A., & Mendell, J. R. (2019). From clinical trials to clinical practice: Practical considerations for gene replacement therapy in SMA type 1. Pediatric Neurology, 100, 3–11. https://doi.org/10.1016/j.pediatrneurol.2019.05.021
- Cunningham, C. J., et al. (2021). Cost-effectiveness and value-based pricing of gene therapies. Journal of Managed Care & Specialty Pharmacy, 27(4), 548–556. https://doi.org/10.18553/jmcp.2021.27.4.548
- O’Connor, B. J., & Larouche, C. (2020). Real-world data for gene therapies: Advancing evidence generation and patient outcomes. Frontiers in Medicine, 7, 450. https://doi.org/10.3389/fmed.2020.00450
- Giacca, M., & Zacchigna, S. (2012). Virus-mediated gene delivery for human gene therapy. Journal of Controlled Release, 161(2), 377–388. https://doi.org/10.1016/j.jconrel.2011.10.026
- Hanna, E., et al. (2017). Gene therapies development: Slow progress and promising future. Human Gene Therapy, 28(4), 290–300. https://doi.org/10.1089/hum.2016.151
- Ayala, R., et al. (2020). Challenges and considerations for gene therapy development and access. Drug Discovery Today, 25(7), 1121–1132. https://doi.org/10.1016/j.drudis.2020.04.005
- FDA (2021). Framework for advancing regulatory science in gene therapy. U.S. Food and Drug Administration. https://www.fda.gov
- EMA (2020). Guidelines for gene therapies in the European Union. European Medicines Agency. https://www.ema.europa.eu

This article has been prepared with the assistance of AI and reviewed by an editor. For more details, please refer to our Terms and Conditions. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author.





